Chloe Yan Chan, Karl Peterson, Lucas Herzog Bromerchenkel, Maria Dittrich, and Sean Monkman
Chloe Yan Chan, Karl Peterson, M.A.Sc. Candidate, Department of Civil Engineering, University of Toronto, 35 St. George Street, Toronto, ON, Canada, chloe.chan@mail.utoronto.ca
Lucas Herzog Bromerchenkel, Asst. Prof., Department of Civil Engineering, University of Toronto, 35 St. George Street, Toronto, ON, Canada, karl.peterson@utoronto.ca
Maria Dittrich, Undergraduate Fellowship Student, Department of Civil Engineering, University of Toronto, 35 St. George Street, Toronto, ON, lucasherzogb@gmail.com
Sean Monkman Assoc. Prof., Department of Physical and Environmental Sciences, University of Toronto Scarborough, 1265 Military Trail, Toronto ON, mdittrich@utsc.utoronto.ca 5 VP Technology Development, CarbonCure Technologies Inc., 60 Trider Crescent, Dartmouth, NS, smonkman@carboncure.com
ABSTRACT
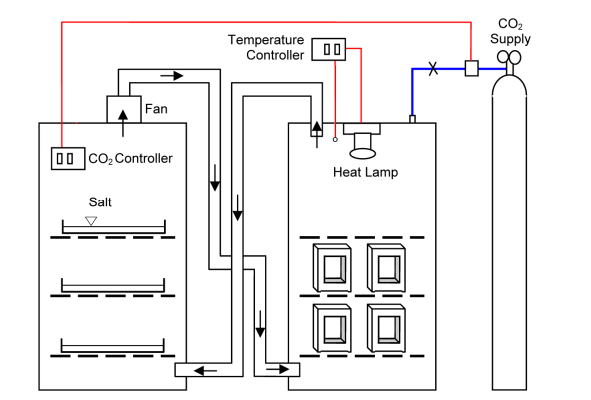
Portland cement based materials have the capacity to capture atmospheric CO2 through carbonation reactions, yielding fixed calcium carbonate phases. In most cases carbonation reactions slowly progress over a timescale of decades before achieving complete carbonation. The ability to rapidly assess the potential CO2 uptake of concrete blocks over their service life would allow for the refinement and selection of manufacturing methods that could optimize CO2 recovery. Industrially produced concrete blocks manufactured under different curing conditions were exposed to a 30 ⁰C, 3% CO2, 55% relative humidity chamber for 2, 4, 8, 16, and 30 days and the degree of carbonation of the cement paste assessed by phenolphthalein staining and petrographic microscope. Carbonation rates were found to be similar for both the CO2-injected and non CO2-injected blocks.
143